Abstract
BACKGROUND: Cytotoxic chemotherapy/agents can cause a range of side effects in cancer patients including anemia, neutropenia and thrombocytopenia, resulting in increased morbidity and mortality. These cytopenias are in part due to massive depletion of bone marrow progenitors. A common target of many chemotherapies is the megakaryocyte (MK), a rare progenitor representing 0.2% of the bone marrow. Loss of MK lineage cells results in thrombocytopenia, therefore, discovering ways to protect MKs from cytotoxic drugs could prevent this life-threatening condition. Here, we show that eltrombopag (EP), a small molecule, non-peptide thrombopoietin receptor (TPO-R) agonist, protects healthy MKs from different cytotoxic agents. We further explored the impact of cytotoxic drugs and EP treatment on signaling molecules active in MKs.
METHODS: Peripheral blood and bone marrow samples were collected from healthy donors after informed consent and following protocols in accordance with the Declaration of Helsinki. CD34+ positive cells were isolated by immuno-magnetic bead separation from the samples and expanded for 8-10 days with a cytokine cocktail to induce MK differentiation. After MK expansion, cells were transferred to 96-well plates pretreated with 14 different drugs including cytarabine, gemcitabine, paclitaxel, carboplatin, cisplatin, doxorubicin, vincristine, etoposide, midostaurin, ruxolitinib, panobinostat, azacitidine, venetoclax and navitoclax. The drugs were plated in 5 different doses in a 10,000-fold concentration range, and cells added with or without EP. After 3 days incubation, the cells were analyzed on a high throughput flow cytometer using Annexin V and 7AAD to distinguish live from dead cells. To understand the impact of the drugs and EP on signaling molecules downstream of TPO-R, we analyzed phosphorylation of AKT, ERK, STAT3 and STAT5 in populations defined by MK markers CD41a, CD42b and CD110 (TPO-R).
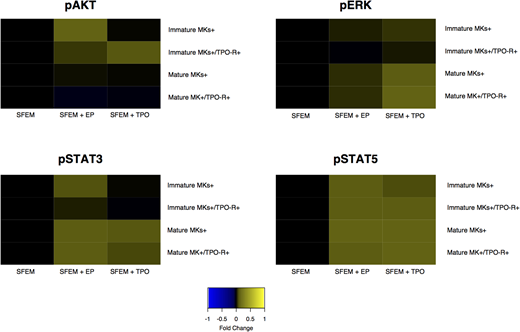
RESULTS: We found that EP overall supports MK survival in the presence of cytotoxic agents. The greatest net effect was observed when EP was combined with BCL2 inhibitors (venetoclax, navitoclax), which resulted in increased MK cell maturation compared to inhibitor alone, while gemcitabine plus EP showed the next best response. EP combined with etoposide, vincristine, cytarabine, paclitaxel, cisplatin and ruxolitinib resulted in an increase of immature MKs without a reduction in mature MK cell numbers. Higher numbers of MKs were observed when EP was combined with midostaurin, carboplatin, panobinostat and doxorubicin, compared to treatment without EP; although these numbers were lower than other tested drugs. Phosphoflow analysis revealed intriguing differences in EP-induced signaling compared to signaling induced by recombinant human thrombopoietin (rhTPO) (Figure 1). Also, EP-induced signaling did not correlate with TPO-R expression. EP activated AKT in all MK subsets, but rhTPO induced AKT only in immature MKs. While rhTPO induced ERK phosphorylation in different MK subsets, EP had no effect on ERK activation. Furthermore, rhTPO activated STAT3 only in mature MKs, but EP induced STAT3 in all MK populations. In contrast, both EP and rhTPO induced phosphorylation of STAT5 in all MK subsets. Overall signaling was inhibited by a selected set of agents (venetoclax, etoposide, midostaurin) compared to basal signaling, but addition of EP prevented the inhibition. Similar to the cell survival analysis, venetoclax combined with EP resulted in complete recovery of signaling activity compared to venetoclax alone. But the response was not as striking for etoposide or midostaurin combination compared to treatment alone.
CONCLUSION: Using MKs expanded from CD34+ cells, we found that EP supports MK survival in the presence of several different cytotoxic agents, with the most striking rescue observed when EP was combined with BCL2 inhibitors. Importantly, EP and rhTPO induced distinct signaling patterns, with EP signaling independent of TPO-R expression, suggesting alternate EP targets. The tested drugs inhibited basal signaling in MKs, but this inhibition was prevented by EP. These results suggest addition of EP to cancer treatment regimens may prevent the inhibitory effects of some agents on MKs and support future investigations to demonstrate clinical efficacy of EP in combinations with some of these cytotoxic agents to alleviate thrombocytopenia.
Javarappa:Novartis Pharma AG: Research Funding. Tsallos:Novartis: Research Funding. Marques Ramos:Novartis: Employment. Pallaud:Novartis: Employment. Heckman:Celgene: Research Funding; Novartis: Research Funding; Orion Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.